Key Takeaways
- Digitally enabled patient engagement and SAE reporting using Foxo Patient Connect
- Replaced fragmented email and manual recruitment spreadsheets with structured workflows
- Streamlined communication across investigators, coordinators, and sponsor teams
- Enhanced audit-readiness through logged, time-stamped communication
- Reduced coordinator administrative burden and improved CRA satisfaction
Customer Challenges
- Email and SMS-based patient contact lacked audit trails and delayed SAE reporting
- Disconnected spreadsheets used for pre-screening and recruitment
- High burden of documentation and manual case tracking
- Limited transparency and efficiency in sponsor query resolution
- Growing multi-site operations demanded a unified digital collaboration hub
Solution
Life Clinical Research, committed to operational excellence and innovation in trial delivery, adopted Foxo across its site network to power a fully paperless, participant-centric trial experience.
With the support of Foxo’s clinical implementation team, LCR deployed key Foxo modules, including Patient Connect and Powerlist, across its clinical research operations. The deployment aligned with the site’s use of Clinical.ly for eSource and eRegulatory, delivering a fully integrated and auditable digital workflow.
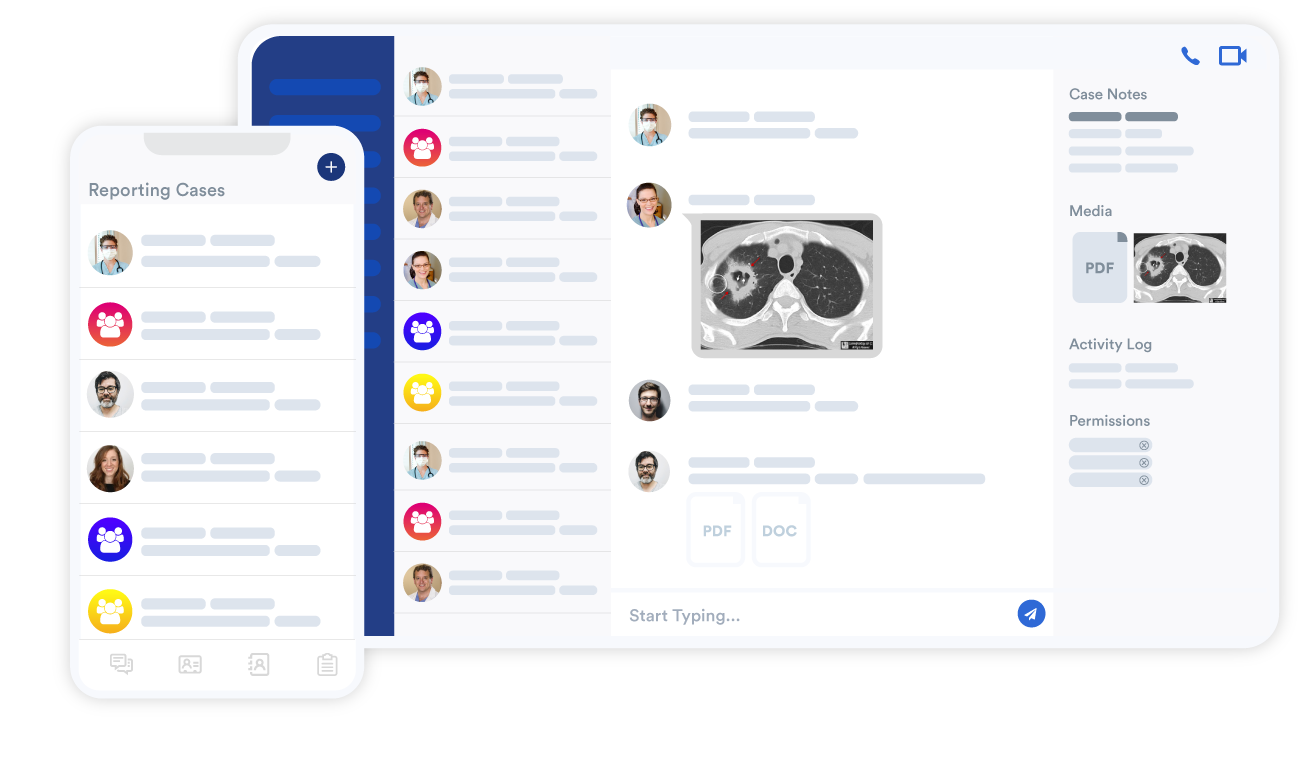
Foxo Patient Connect allows participants to securely message the study team by scanning a QR code provided on a branded patient card with no downloads or onboarding required. Participants can report adverse events, submit photographs, ask medication questions, or request reschedule appointments. Messages are time-stamped, securely logged, and automatically escalated if not acknowledged within five minutes, ensuring swift and compliant responses.
Foxo Powerlist transformed the way LCR manages pre-screening. Investigators flag potential participants by generating a digital Participant Card and placing it into a study-specific team chat. Coordinators can then review, tag, and triage candidates collaboratively - streamlining lead generation and providing sponsors with real-time recruitment visibility.
Looking ahead, Powerlist offers potential for expansion via Foxo’s Referrer Connect, enabling external referrers - such as GPs or specialists - to submit trial candidates directly into the Powerlist workflow without requiring a Foxo account or onboarding. This future-facing feature mirrors the seamless functionality of Patient Connect and could unlock new recruitment pathways for LCR by capturing inbound referrals from trusted healthcare providers in real time.
Use cases
- QR-enabled secure patient messaging via Foxo Patient Connect
- Real-time SAE reporting with escalation protocols
- Powerlist-driven pre-screening, tagging, and adjudication workflows
- Streamlined sponsor communication and response tracking
- Investigator-to-coordinator messaging in patient context
Benefits
- 100% paperless operations with integrated audit logs
- Minimised SAE reporting delays via real-time, patient-initiated messaging
- Clear visibility of recruitment pipelines and pre-screening decisions
- Improved sponsor and CRA engagement
- Eliminated non-compliant tools (e.g. unsecured SMS, email)